- HOME
- WHY LUMASON
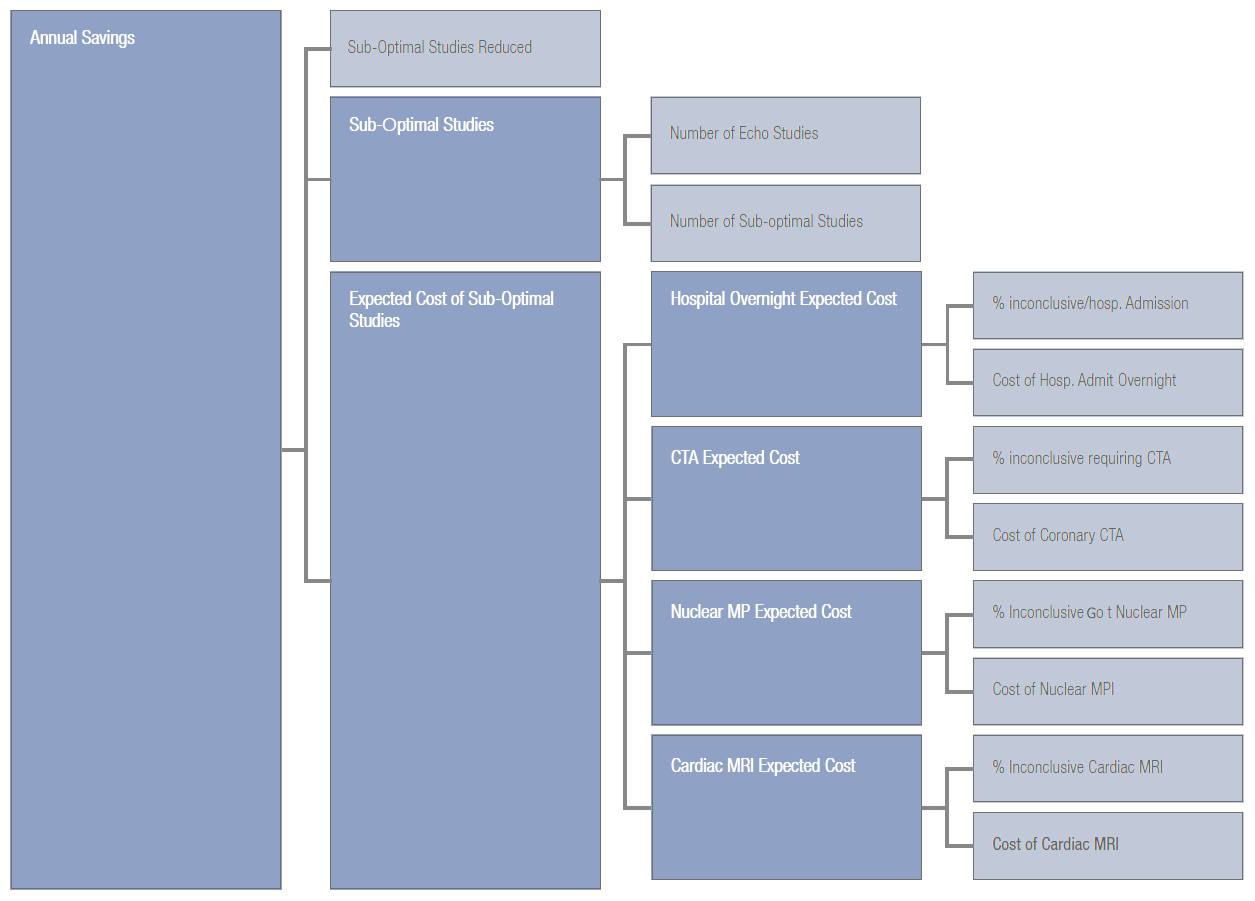
- HEALTH ECONOMICS
- SUPPORT
- PLAY WITH US

Becoming a Sonographer?
Already a Sonographer?Improve your ability to quickly and accurately identify apical views and wall segments of the heart.
A Day in the Life of a Sonographer
Your work life is hectic and full of decisions that can impact your every day. Experience a wide array of decisions and outcomes together with helpful tips to improve your day. - Find a Rep